Recently, the Quality and Safety Research Group at the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences (CAAS), developed a series of nickel–iron (Ni–Fe) bimetallic metal-organic frameworks (MOFs) by precisely tuning the Ni/Fe molar ratio. These materials demonstrated rapid, efficient, and selective adsorption of both single and binary organic dye pollutants, offering an innovative solution for the remediation of agricultural non-point source pollution. The study, titled “Analysis of Microstructure Properties of Ni–Fe MOFs and Their Influence on Selective Recognition of Single and Binary Dye Systems,” was published in npj Clean Water (IF= 10.5, Q1).
Pollutants such as pesticides, heavy metals, and organic dyes from agriculture, aquaculture, and rural domestic wastewater pose serious threats to water safety and the quality of agricultural products. Conventional adsorbents like activated carbon and silica gel suffer from low adsorption capacity, poor selectivity, and limited reusability, making them inadequate for treating complex pollution systems. While metal-organic frameworks (MOFs) offer advantages such as high surface area and tunable porosity, single-metal MOFs still face limitations in selectivity and stability when applied to complex wastewater. In contrast, bimetallic MOFs provide a promising strategy to overcome these challenges through synergistic interactions between metal components.
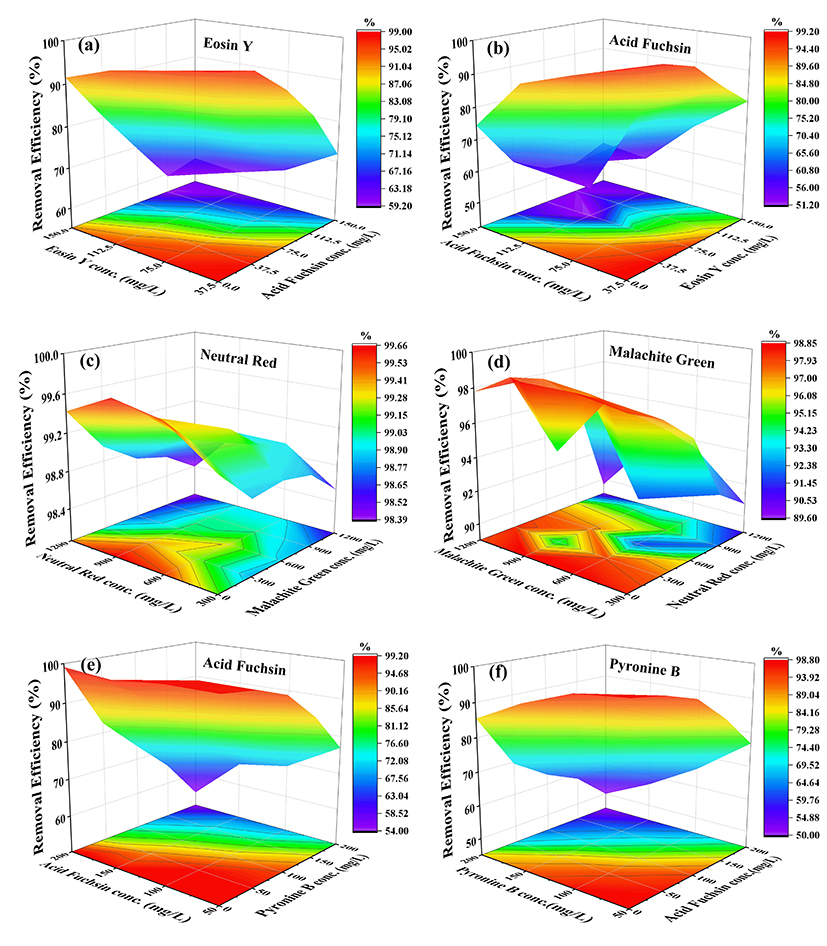
Fig. 1. Adsorption efficiency of different MOFs in binary dye systems.
Note: In the EY/AF system, Fe-MOFs adsorption efficiency for EY (a) and AF (b); in the NR/MG system, Ni-MOFs adsorption efficiency for NR (c) and MG (d); in the AF/PB system, Ni/Fe (1:1) MOFs adsorption efficiency for AF (e) and PB (f).
In this study, five MOFs were synthesized via a one-pot solvothermal method by adjusting the Ni/Fe molar ratio: Fe-MOFs, Ni-MOFs, and Ni/Fe MOFs with molar ratios of 3:1, 1:1, and 1:3. These materials were comprehensively characterized using scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), and nitrogen adsorption–desorption (BET) analysis. The results showed that the Ni/Fe ratio significantly influenced both microstructure and adsorption performance. As the Ni/Fe ratio increased, the material morphology shifted from nanospheres (Fe-MOFs) to flower-like structures (Ni/Fe MOFs) to rhomboid blocks (Ni-MOFs), while the surface area decreased from 281.3 m²/g (Fe-MOFs) to 67.11 m²/g (Ni/Fe (1:1)) and further to 0.5369 m²/g (Ni-MOFs). XPS and FT-IR analyses confirmed the formation of stable bimetallic clusters via synergistic coordination between Ni²⁺ and Fe²⁺/Fe³⁺, with precisely tunable surface charge and functional group distributions.
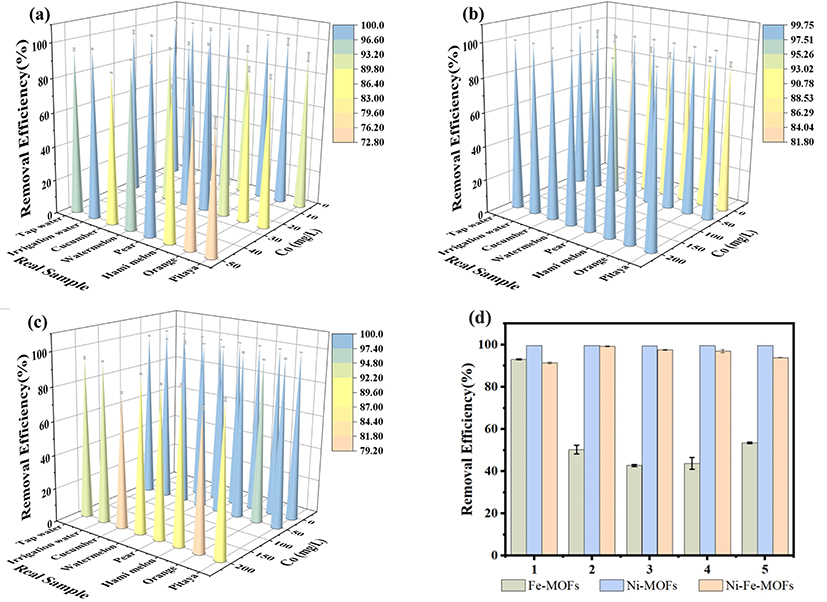
Fig. 2. Performance of different MOFs in real sample applications (a–c) and reusability tests (d).
Note: (a) EY adsorption by Fe-MOFs in real samples; (b) NR adsorption by Ni-MOFs in real samples; (c) AF adsorption by Ni/Fe (1:1) MOFs in real samples; (d) Reusability performance of different MOFs.
Adsorption experiments using eight representative dyes demonstrated that each MOF exhibited selective adsorption behavior. Three dye systems were selected for further investigation. Mechanistic studies revealed that Fe-MOFs relied on electrostatic interactions via positively charged Fe³⁺ surfaces (Zeta potential: –6.71 mV), Ni-MOFs achieved selectivity through size exclusion via micropores (with pore size reduced from 44.78 nm to 17.61 nm), and bimetallic MOFs utilized redox-active Ni–Fe–O clusters for chemical bonding. Notably, in binary dye systems, Fe-MOFs showed competitive adsorption for EY and AF, Ni-MOFs exhibited synergistic adsorption for NR and MG, and Ni/Fe (1:1) MOFs demonstrated competitive adsorption for AF and PB—further highlighting the effect of structural tuning on adsorption behavior.
The Institute of Vegetables and Flowers at the Chinese Academy of Agricultural Sciences is the lead institution for this work. Dan Xu (MSc, jointly trained by Yantai University), Associate Professor Xiaolin Cao (Yantai University), and Dr. Jie Zhou are co-first authors. Professor Guangyang Liu, Professor Jing Wang (from the Institute of Quality Standards), and Professor Donghui Xu are co-corresponding authors. The study was conducted under the support of the National Key Laboratory of Vegetable Biological Breeding and the Key Laboratory of Vegetable Quality and Safety Control of the Ministry of Agriculture and Rural Affairs, and funded by the National Key R&D Program, the National Vegetable Industry Technology System, the Beijing Natural Science Foundation, and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences..
Web site of the paper: https://link.springer.com/article/10.1038/s41545-025-00470-6