A synonymous mutation is a change in the coding DNA sequence that does not alter the encoded amino acid. Compared with non-synonymous mutations, which change protein sequences, it has long been thought that most synonymous mutations are biologically silent. Studies showed that some synonymous positions may influence several transcriptional and posttranscriptional processes, including mRNA splicing, tRNA selection, and translation efficiency. Although these findings suggest that synonymous changes play a role in cellular function and organismal fitness, there is nonetheless little genetic evidence in multicellular organisms of synonymous mutations shaping biological traits, or the associated modes of action. N6-Methyladenosine RNA (m6A) is a prevalent messenger RNA modification in eukaryotes. m6A has been demonstrated to regulate multiple post-transcriptional processes, including mRNA stability and translation. However, direct genetic evidence linking m6A to specific biological outcomes remains limited.

Recently, A team led by Professor Xueyong Yang from the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Professor Sanwen Huang from the Agricultural Genomics Institute at Shenzhen, Chinese Academy of agricultural Sciences, and Professor Yiliang Ding from the John Inns Centre, UK, published an article entitled "Recessive epistasis of a synonymous confers cucumber domestication through epitranscriptomic regulation " in the international authoritative journal Cell. This study provides genetic evidence for the first time that synonymous mutations can regulate important traits of domestication by altering m6A modifications and mRNA structural conformation.
Fruit length, which is strongly correlated with yield, is a domestication trait. Domesticated cucumber (Cucumis sativus L.) has substantially longer fruit than those of the wild relatives; however, the genetic basis for this trait is not clear. We previously detected a fruit length QTL (quantitative trait locus) on chromosome 1, FL1, which contributes 30% to controlling fruit length. To map the causal gene(s) at the FL1 locus, backcross introgression lines (ILs) were developed in which chromosome segments of wild cucumber (C. sativus var. hardwickii PI183967), which bears short and round fruit, were introduced into cultivated cucumber (C. sativus cv. Xintaimici) that has typical elongated fruit. We discovered two closely linked, epistatically interacting loci associated with fruit length diversity and domestication. Unexpectedly,when the FL1.2C allele was present regardless of the type of FL1.1 allele, fruit length was determined by FL1.2C, indicating that FL1.2C is recessively epistatic to FL1.1. By fixing the FL1.2 locus as wild allele, we screened over 10,000 -individual recombinants and discovered the cryptic variations of FL1.1 that function in a specific genotype.
Further research reveals that FL1.2 encode an aminocyclopropane-1-carboxylic acid synthase (ACS2) that we previously found controls ethylene dose-dependent fruit elongation (The plant cell, 2019). Genomic sequencing of ACS2 in FL1.1W FL1.2W and FL1.1W FL1.2C plants revealed a three-base insertion in the 5' UTR of ACS2W, two SNPs in the introns and three synonymous SNPs in the exons. We finally found that the C>T synonymous mutation at position 1287 of the third exon could alter the protein level of ACS2 by 4 to 5 times. We established an effective CBE base editing system in cucumber to genetically verify the functionality of the C1287 mutation directly. The C to T transition significantly reduced ACS2 protein abundance and promoted fruit elongation, demonstrating that the 1287C>T synonymous mutation is the causative SNP for FL1.2 and fruit length increase during domestication (Figure 1).
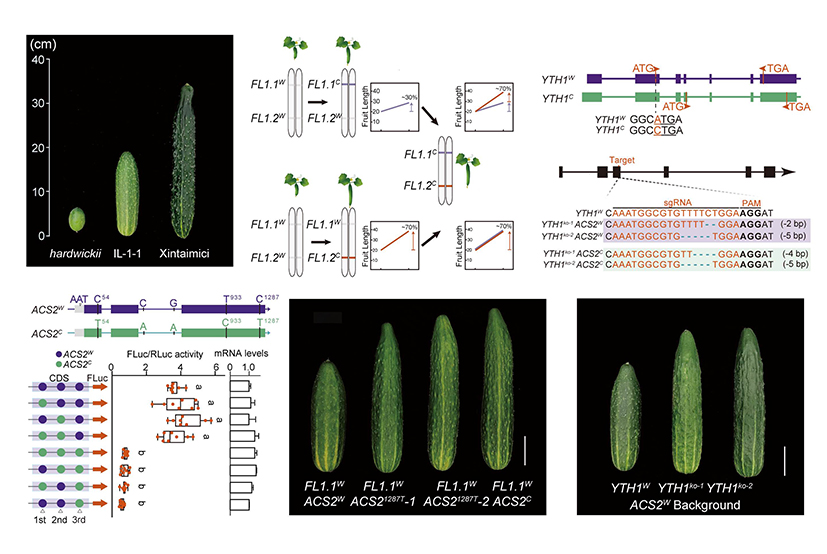
Figure 1. Two epistatically interacting genes contributed to cucumber fruit length domestication.
The gene FL1.1 that interacts with FL1.2 encodes a m6A reading protein, YTH1. We revealed the genetic mechanism by which recessive epistasis of ACS2 to YTH1 confers cucumber fruit length during domestication: In wild cucumber, ACS21287C results in m6A modification on nearby adenosine residues, YTH1 recognizes the m6A modification and increases ACS2 translation efficiency, leads to increased ethylene production and reduced cell division, resulting in shorter fruit. In cultivated cucumber, ACS21287T disrupts m6A methylation leading to attenuated protein production and fruit elongation.
We further analyzed the mechanism by which synonymous mutations regulate the translation efficiency of ACS2, and found that the changes in mRNA structure caused by m6A modification and synonymous mutations determine the translation efficiency of ACS2. By analyzing the diversity of mRNA structural conformations of ACS2 at the single-molecule resolution, we found that compared with ACS21287T, all the structural conformations of ACS21287C showed single-strandedness across the 1287C>T synonymous mutation site. The m6A modification further alters the relatively more compact RNA structural conformation into weakest RNA structural conformation. Therefore, m6A may maintain the mRNA structural equilibrium by stabilizing and regulating conformational transition (Figure 2).
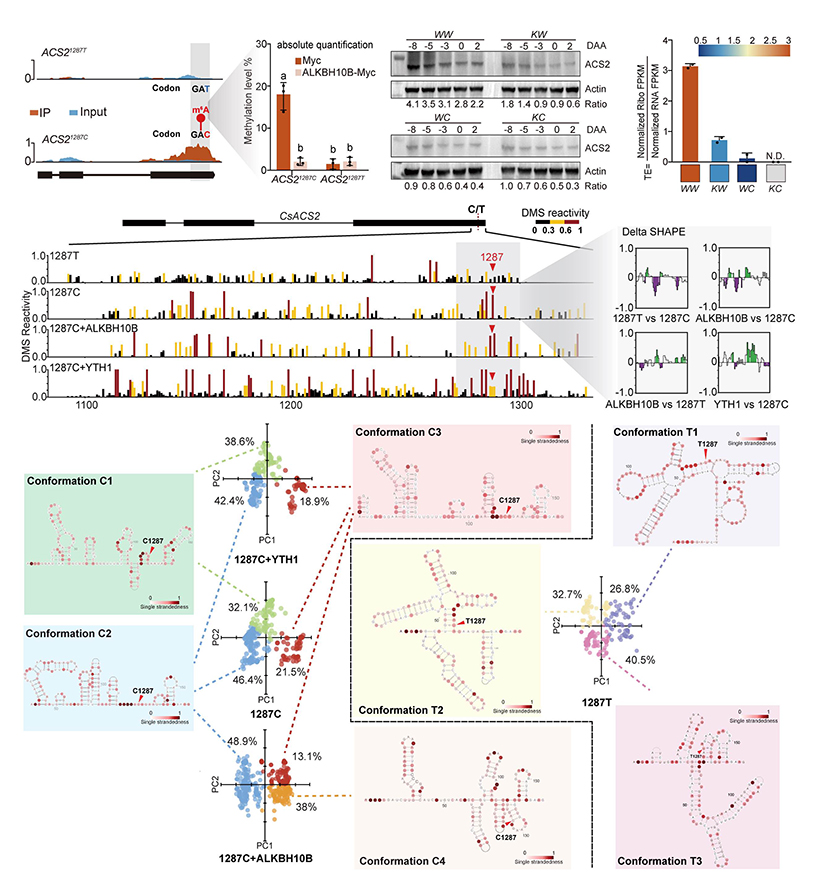
Figure 2. The 1287C>T synonymous mutation changes the translation efficiency of ACS2 via m6A modification and RNA structural alterations
Notably, a recent study published on Cell found that a category of synonymous mutations can perturb m6A modification patterns and promote tumorigenesis through mRNA expression regulation.
Our study provides an organismal example of synonymous mutation controlling domesticated traits through m6A modification and RNA structure-mediated epitranscriptomic regulations,suggesting an important function of synonymous mutations that has long been overlooked. These findings suggest that synonymous mutations could be selected during domestication to alter post-transcriptional gene expression such as translation efficiency in response to selective pressures. This challenges the traditional view and highlights the potential importance of synonymous mutations in crop improvement strategies.
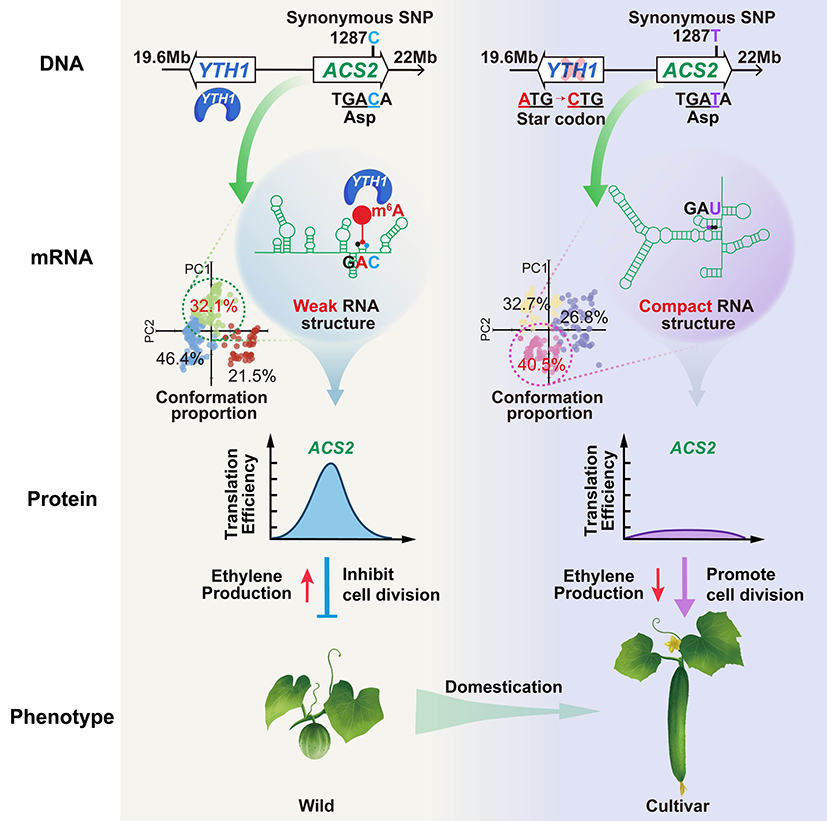
Figure 3. The molecular mechanism by which synonymous mutations regulate the fruit length domestication
Professor Xueyong Yang from the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Professor Sanwen Huang from the Agricultural Genomics Institute at Shenzhen, Chinese Academy of agricultural Sciences, and Professor Yiliang Ding from the John Inns Centre, UK, are the co-corresponding authors of this paper. Dr. Tongxu Xin from the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Associate Professor Zhen Zhang from Henan University, Dr. Yueying Zhang from the John Innas Centre in the UK, and Xutong Li, a graduate master's student from the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, are the co-first authors of this article. The Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, is the first author's institution and the corresponding author's institution of the paper. Professor Chuan He from the University of Chicago provided valuable opinions and technical support for this research work. This research was supported by the National Natural Science Foundation of China, the National Key Research and Development Program of China, and the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences.
Link:https://www.cell.com/cell/fulltext/S0092-8674(25)00674-9